CTTI’s interactive database was developed as a tool to identify, collect, and organize feasibility studies using mobile technologies to capture data in clinical research, intended to serve as a dynamic, up to date repository. With this database, CTTI intends to help alleviate future development of redundant studies and contribute to the design and implementation of high quality, efficient trials.
The information can be used by investigators in the field to make decisions regarding which technology would be most useful for their research, by sponsors to support trial design, and may also be useful to researchers, patient advocacy groups, technology manufacturers, regulatory bodies, and ethicists for a variety of purposes, as laid out in the “Who should use” tab.
This database was created by a multi-stakeholder project team convened by CTTI. Original content was derived from a systematic search of scientific literature indexed in PubMed and published between January 2014 and May 2019. Additional content will be reviewed and added regularly.
Step 1: Type the research topic in “search the database”

Step 2: Select “Filter.” Categories will appear including Year, Technology, Concept, Therapeutic Area, etc.
Step 3: A drop down menu will appear for each category. Select the topic for each category (Ex. Year: 2015, Category: Operational Feasibility, Concept: Behavior Monitoring, etc.)

Step 4: Select the columns that the user would like to display (Can only select up to 8 columns). A list of publications will appear separated by the selected columns.
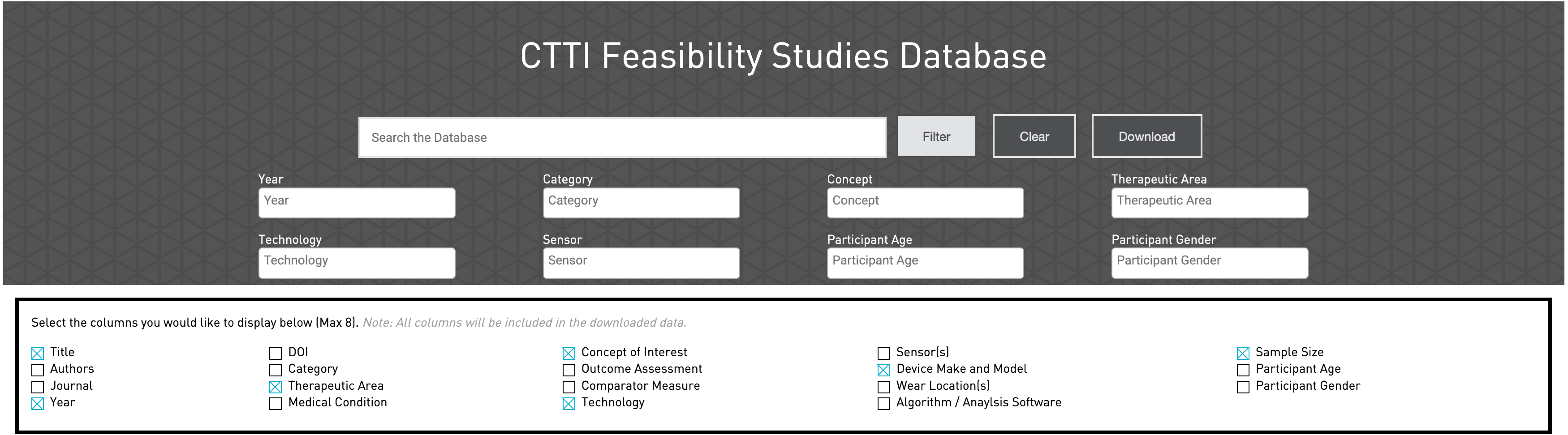
Table 1: Systematic review inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|